Gerbig Certification Division is part of CSI Testing to become the largest independent certification provider in the upper Midwest — now serving customers under the CSI Testing name.
You can still receive the exceptional testing standards, expertise, and reliability you’ve come to rely on from Gerbig Certification, just in a new place. Visit CSI Testing’s website to learn more. Gerbig is still your trusted partner for the construction of cleanrooms, manufacturing, and filter providers.
Cleanroom Certification & Validation Services
Gerbig Cleanrooms is one of the leading cleanroom validation companies in the country with over 30 years of cleanroom testing experience. Gerbig is a National Environmental Balancing Bureau (NEBB) accredited testing firm using highly trained technicians who can perform the necessary validation procedure services to meet cleanroom classification and certification requirements. Gerbig provides cleanroom air filter repairs and replacements. Service to HEPA filters is critical to maintaining ISO cleanroom certification, keeping manufacturing cleanrooms from ISO 3 to ISO 9. Our expertise in validating cleanroom classes, ranging from industrial to scientific, makes Gerbig a preferred source for cleanroom certification services.
Gerbig cleanroom testing is in accordance with:
- • International Organization for Standardization (ISO)
• Institute of Environmental Science and Technology (IEST)
• National Science Foundation (NSF)
• Controlled Environmental Testing Association (CETA)
• Current Good Manufacturing Practices (cGMP)
Gerbig Offers:
- Cleanroom Testing & Certification
BSC Certification & Services
Environmental Testing
Cleanbench Testing
Decontamination Services
Fume Hood
USP 797/800
Cleanroom Evaluations & Audits
cGMP Testing
Find a Solution Today
Why Validate Your Cleanroom?
The cleanroom validation procedure is documented evidence that proves a system will produce a product that meets all specifications and standards. Each cleanroom needs to meet a certain amount of class standards, set by The National Environmental Balancing Bureau (NEBB), to be considered compliant and qualified for use. The facility using the system is solely responsible for validation. While it is common for a facility to contract an outside firm to perform this complex service, it is still the facility’s obligation to approve protocols and execute testing.
Validation is FDA-mandated for the pharmaceutical, biopharmaceutical, medical device, and food manufacturing industries. Although cleanroom certification is required, there are many reasons to have your cleanroom certified.
The cleanroom validation procedure ensures that the facility, equipment and environment:
- Are fit for the cleanroom’s intended purpose
- Meet User Requirement Specifications (URS)
- Meet defined regulatory requirements
- Function together as a system to meet standards
Primary Cleanroom Tests
Tests are recommended for all cleanrooms:
- ISO 14644-1 particle counts
- Velocities & air volume
- Temperature & humidity uniformity
- Room air change rates
- Pressure differentials
- HEPA filter integrity testing
- Temperature & RH
- Static electricity evaluation/dissipation testing
Optional cleanroom services include:
- Particle recovery
- Airflow visualization smoke test
- BSC testing & decontamination
- Fume hoods
- Light and/or sound level testing
- Laminarity
- Clean benches
Cleanroom environmental monitoring:
Ongoing periodic monitoring for microbial and other key controlled environment attributes:
- Airborne viable organisms
- Surface viable organisms
- Airborne particle counts
Cleanroom Testing Frequency
Your certification schedule will depend on your cleanroom class. You may need monthly, annual, or biennial checks. No matter the case, it’s important to stay up to date to avoid costly problems later.
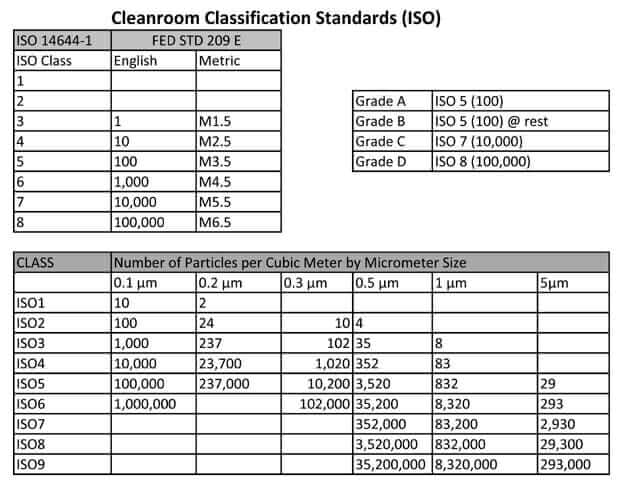
ISO 14644-1 & FS 209E Class Limits